COGNOA RECEIVES FDA MARKETING AUTHORIZATION FOR FIRST-OF-ITS-KIND AUTISM DIAGNOSIS AID
The U.S. Food and Drug Administration has authorized marketing of a device to help diagnose autism spectrum disorder (ASD). The Cognoa ASD Diagnosis Aid is a machine learning-based software intended to help health care providers diagnose ASD in children 18 months through 5 years of age who exhibit potential symptoms of the disorder.
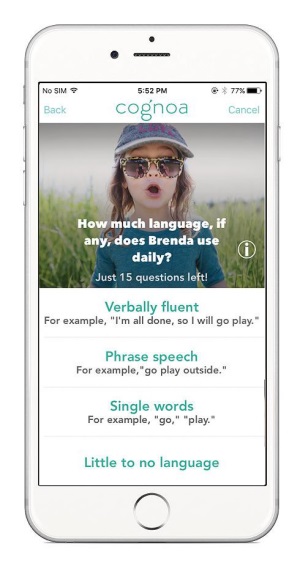
NEW PATH FORWARD: The ASD diagnostic tool is designed to help primary care clinicians and pediatricians evaluate and diagnose suspected cases of autism among children.
"Autism spectrum disorder can delay a child's physical, cognitive and social development, including motor skill development, learning, communication and interacting with others. The earlier ASD can be diagnosed, the more quickly intervention strategies and appropriate therapies can begin," said Jeff Shuren, M.D., J.D., director of the FDA's Center for Devices and Radiological Health. "Today's marketing authorization provides a new tool for helping diagnose children with ASD."
The CDC and Prevention defines ASD as a “developmental disability that can cause significant social, communication and behavioral challenges” and is estimated to affect about 1 in 54 children. While ASD may be detected as early as 18 months old, many children are not diagnosed until later in childhood, which can delay treatment and early intervention. The average age of diagnosis for ASD is 4.3 years. Some delays in diagnosis are due to the need for children to be referred to specialists with expertise in ASD.
The Cognoa ASD Diagnosis Aid is a software as a medical device that uses a machine learning algorithm to receive input from parents or caregivers, video analysts and health care providers to assist physicians evaluate a patient at risk of ASD. The device consists of three main components: a mobile app for caregivers and parents to answer questions about behavior problems and to upload videos of their child; a video analysis portal that allows manufacturer-trained and certified specialists to view and analyze uploaded videos of patients; and a health care provider portal that is intended for a health care provider to enter answers to preloaded questions about behavior problems, track the information provided by parents or caregivers and review a report of the results. After processing the information provided by parents, caregivers and healthcare providers, the ASD Diagnosis Aid reports a positive or negative diagnosis if there is sufficient information for
its algorithm to make a diagnosis. If there is insufficient information to render a "Positive for ASD" or "Negative for ASD" result to help determine a diagnosis, the ASD Diagnosis Aid will report that no result can be generated.
The FDA assessed the safety and effectiveness of the Cognoa ASD Diagnosis Aid in a study of 425 patients aged 18 months through 5 years in 14 different clinical care sites, with an average age of 2.8 years. The study compared the assessments made by the device directly against the assessments made by a panel of clinical experts who used the current standard ASD diagnostic process. The device provided a "Positive for ASD" or "Negative for ASD" result to aid in making a diagnosis in 32% of patients. For those with a "Positive for ASD" or "Negative for ASD" result, the device results matched the panel's conclusions for 81% of patients who tested positive for ASD by the device and 98% of patients who tested negative for ASD by the device. In addition, the device made an accurate ASD determination in 98.4% of patients with the condition and in 78.9% of patients without the condition.
The risks associated with the use of the device include misdiagnosis and delayed diagnosis of ASD, based on a false positive result (observed in 15 out of 303 study subjects without ASD), a false negative result (observed in one out of 122 study subjects with ASD) or when no result was generated. Both misdiagnosis or missed diagnosis can result in delayed treatment of ASD and delivery of treatment not appropriate for ASD.
The Cognoa ASD Diagnosis Aid is indicated as an aid in the diagnosis of ASD for patients 18 months through 5 years of age who are at risk of developmental delay based on concerns of a parent, caregiver, or health care provider. The device is not indicated for use as a stand-alone diagnostic device but as an adjunct to the diagnostic process. Lean more at canvasdx.com
ABOUT THE FOOD AND DRUG ADMINISTRATION
The FDA, an agency within the U.S. Department of Health and Human Services, protects the public health by assuring the safety, effectiveness, and security of human and veterinary drugs, vaccines and other biological products for human use, and medical devices. The agency also is responsible for the safety and security of our nation's food supply, cosmetics, dietary supplements, products that give off electronic radiation, and for regulating tobacco products. Visit fda.gov