People with rare diseases often describe itself as a community – one that provides a tremendous amount of mutual support, guidance and advice. The ORDR can be a respected starting point for diving into the various programs and organizations provided by the U.S. specifically for rare disease patients and their families.
NTRODUCTION
While rare diseases are rare, individually, they affect anywhere from 25-30 million individuals in the United States all together. Collectively, rare diseases aren't rare. There are more than 7,000 rare disorders. Many affect children, and many are genetic. Many rare diseases affect multiple systems of the body and present significant challenges for individuals and families.
The Office of Rare Diseases Research (ORDR) was first established in 1987 as the Office of Rare Diseases, and then as the Office of Rare Diseases Research after it was formerly established in statute by the Rare Diseases Act of 2002. The ORDR is part of the National Institutes of Health (NIH), the United States' medical research agency, and specifically of the National Center for Advancing Translational Sciences (NCATS). The NIH works to improve health and save lives. The ORDR "coordinates and supports rare disease research, responds to research opportunities for rare diseases, and provides information on rare diseases."
The ORDR offers a wealth of information and support for families and individuals affected by rare diseases. Receiving a diagnosis of a rare disease is a shock. Often, there is limited information and sometimes even the diagnosing physicians have little knowledge of the condition. Parents and families can feel cast adrif with little understanding or guidance. Attempting to find reliable information on the Internet can seem almost impossible as a search may bring back a torrent of information, leaving the family inundated and overwhelmed.
"There is much hope for rare diseases. Our understanding of what causes many of these disorders is unprecedented, and new treatment approaches hold great promise. Through collaboration with patients, families and our other stakeholders, NCATS is helping to bring more treatments to more rare diseases patients more quickly." Petra Kaufmann, M.D., M.Sc., director of the Office of Rare Diseases Research at NCATS and NIH.
THE GENETIC AND RARE DISEASES INFORMATION CENTER (GARD)
INFORMATION CENTER (GARD) One of the main ways that the ORDR helps patients and families navigate the new (rare disease) world they've entered is through the Genetic and Rare Diseases Information Center (GARD), which was created in 2001. In 2008, the GARD website was launched. Overseen by the ORDR, this informational website provides families with current, reliable and easy-to-understand information about rare or genetic diseases. To accommodate an international community, some information is available in English and Spanish. One unique challenge of dealing with rare diseases is the lack of reliable information, not just for affected families, but for the physicians who treat these patients. Unless a person with a rare disease is being seen at a special clinic, center of excellence or by an expert in a specific rare disease, they are often seen by a primary care physician or pediatrician who has little familiarity with the disorder. To make informed and educated decisions about their or their child's health, in-depth, understandable medical information is needed. Depending on the source, the average amount of time to a rare disease diagnosis is anywhere from 5-7 years after symptoms begin. The ability of patients to garner good information may help to reduce the delay in diagnosis and enables them to have more productive meetings with physicians after a diagnosis is made.
ORDR Provides Summaries on Many of the 7,000 Known Rare
Diseases: The information can be viewed within the diseases section of GARD's website. Users can browse by the first letter of the rare disease, by the category in which it falls or by conducting a search by the rare disease name. A disease page contains a wealth of information including a summary of the rare disease as well as information about the symptoms, cause, diagnosis, and treatments. The information is taken out of the complex medical terminology and presented in an understandable manner as possible. It also provides links to reliable resources or organizations associated with the rare disease who may be able to shed more light on the condition that could potentially lead to further assis tance, identifying a patient advocacy organization that has a variety of services and programs can provide a place to engage with others going through the same experience. Every report includes a link to current news and events for that specific rare disease. This can include NIH-sponsored conferences and other programs that they provide to the rare disease patient community. Included is a reference list with a bibliography indicating the materials used to create the report. These may include medical journal articles, abstracts, and other links to online references. The publications mentioned in the reference section are usually not written in layman's terms, thus will appear to be very technical in nature.
A unique feature of the website is Your Questions Answered. This is a listing of questions that families and others have asked about a particular rare disease and that have been answered by the GARD staff. These questions are added to the disease page. This unit is more than a traditional Frequently Asked Questions (FAQ) section, and can be extremely beneficial to those seeking specific information. The questions are often based on parental and personal experience and may more closely resemble a family's issues.
Need information that will help to manage day-to-day challenges and that could enable you to have a better quality of life? GARD's specialty prepared guides (rarediseases.info.nih.gov/guides) are a series that cover general topics and provide key information and answers for patients and families seeking lifestyle modifications. Topics include How to Find a Disease Specialist, Tips for Finding Financial Aid, and Help with Travel Costs. These guides provide information that can apply to patients and fami lies who share common issues although they do not share the same diagnosis. There are also guides to help researchers, healthcare professionals, and teachers or students.
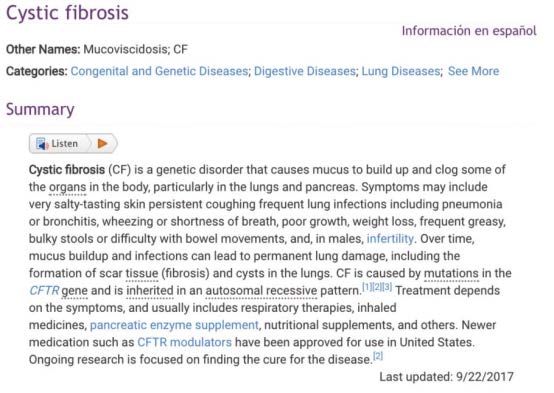
FINDING THE WAY: A selection from the introductory paragraph for the cystic fibrosis GARD disease page found at rarediseases.info.nih.gov/diseases/6233/cystic-fibrosis
Information Specialists:
In addition to the tremendous amount of information provided through the GARD website, they also have Information Specialists on staff who can speak with patients and families. Oftentimes, the ever-increasing technological advances can lead associations and organizations to limit or, in some cases, forgo the option of human interaction. Thankfully, GARD still provides a visitor to their website, the ability to talk to a person. People can contact GARD via email, a form on the website, or by calling and leaving a message. They will be contacted back (usually the same day). Despite the growing familiarity and ease of using technology and the Internet, the ability to interact with an actual person can dramatically save time, effort, and frustration. Information Specialists are available via:
• By Telephone - Monday - Friday, 12-6 p.m. Eastern Time Toll-free: 888-205-2311 TTY: 888-205-3223 International: 301-251-4925 or 240-439-8429
• By U.S. Mail or Fax - Answered within 12 business days Genetic and Rare Diseases Information Center P.O. Box 8126, Gaithersburg, MD 20898-8126 Fax: 301-251-4911
• By E-mail Form Visit: rarediseases.info.nih.gov/about-gard/contact-gard
Information specialists can provide medical information in easy-to-understand terminology, as well as information about genetic testing and services, patient advocacy organizations, medical journal articles that apply to an individual or family, and how to find and access research studies and clinical trials. They can help people locate and understand other resources that may be applicable to their needs. However, it is important to know that information specialists cannot give medical advice or attempt to provide a diagnosis.
Providing recommendations are an important aspect of what the ORDR does. One of the trickiest aspects of searching the Internet for medical information is how to determine whether a website is credible or not. Fortunately, the ORDR has already done the legwork and is considered a trusted resource. Individuals or families can receive, whether directly from the GARD website or by contacting one of its Information Specialists, recommendations for organizations whose missions are to provide support or further services to individuals, families, caregivers and even healthcare providers. This can save a tremendous amount of time and avoid a lot of aggravation for families and patients. Most sections of the GARD website clearly indicate where to find recommendations to other helpful, reliable sources of information.
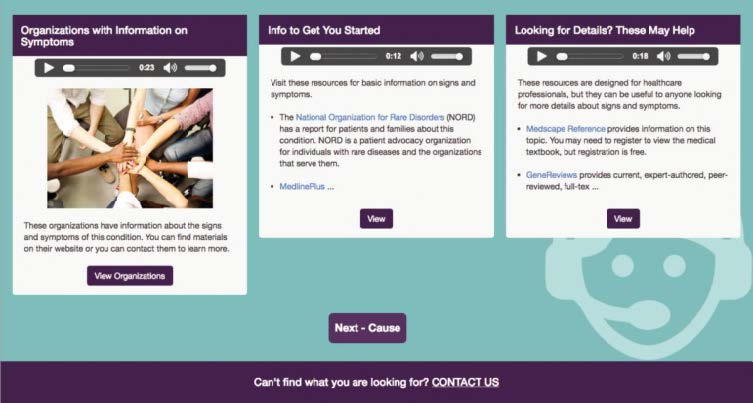
GETTING ANSWERS: A selection from the Keeping Up-to-Date page for Huntington Disease found at rarediseases.info.nih.gov/diseases/6677/huntington-disease#diseaseLivingWithSection
The GARD Information Navigator:
Another interesting feature of the website is the GARD Information Navigator (rarediseases.info.nih.gov/diseases/gard-information-navigator). These services launch an application that allows the user to select a specific topic for a specific disease such as diagnosis or treatments. The user will receive a custom page detailing the topic chosen. For example, a user could select the disorder Huntington disease and pick "causes" and then "keeping up-to-date." The Navigator will return a custom page on causes and then a custom page on the most recent developments concerning the disease.
ORPHAN DRUGS
The Orphan Drug Act, enacted in 1983, gave pharmaceutical companies several incentives to develop treatments for very small patient populations. Before its passage, 34 drugs were approved. After its passage, there have been more than 600 drug indications from more than 450 distinct drug products
The GARD website offers a searchable list of orphan drugs approved by the Food and Drug Administration (FDA) [raredis- eases.info.nih.gov/diseases/fda-orphan-drugs/]. The list can be browsed by disease and there are links to the FDA and other websites that have more detailed information.
RESEARCH
Supporting research is a key aspect of what the ORDR does – the word research is in its name. The GARD website provides a video titled How to Get Involved in Research. The video discusses the best ways to find clinical trials for rare and genetic diseases and provides some very basic and general information about clinical trials, and what it takes to participate in one.
In addition, the NCATS ORDR has worked with patient groups and other stakeholders to develop a Toolkit for Patient-Focused Therapy Development (ncats.nih.gov/toolkit), which is part of the GARD website. It was created to provide a collection of online resources that can help patient groups find the tools they need to advance towards the development of better treatments for their disease. Launched in September 2017, the toolkit provides a centralized portal with tools that include how to establish a registry, how to drive rare diseases research, how to work with the NIH and FDA, and how to support post-market surveillance for approved therapies.
Clinical Trials
Clinical trials can potentially be vitally important to families affected by rare diseases. Sometimes, these studies are for treatments for diseases that otherwise have no effective therapies. When clinical trials are successful, they are often big news in the media, but what about before they are successful? Should parents consider putting their children in a trial? The ORDR provides links to organizations that can educate individuals and families about clinical trials including discussing the pros and cons of participation in order to be better informed about one's medical condition
The ORDR also coordinates collaborative research efforts toward rare diseases, which includes providing support to medical institutes and centers. The organization also fosters collaboration among physicians, patients and families, patient advocacy organizations, research clinical center personnel and other stakeholders or people interested in rare disease research.
Another unique problem for rare diseases is sometimes there are no physicians or researchers investigating a disease. Imagine not only receiving a diagnosis of a rare disease, but discovering no one is even studying the disease to learn what causes it or what might be an effective treatment. The ORDR supports the training of NIH rare disease investigators. It works with the Institutes and Centers that make up the NIH at the NIH Clinical Center hospital and medical research centers throughout the country to support these investigators.
The ORDR, together with ten other NIH Institutes and Centers, also oversees the Rare Diseases Clinical Research Network (RDCRN) [rarediseasesnetwork.org]. This Network is made up of more than 21 disease research groups called consortia. Each consortium covers a type of disorder such as dystonia or inherited neuropathies. Among the many diseases that are covered include Parkinson disease with autonomic failure, amyotrophic later sclerosis, progressive muscular atrophy, osteogenesis imperfecta, Collectively, and Behcet's disease. these consortia conduct research on almost 200 different disorders and exist as a collaborative network between clinical investigators and patient organizations. Patients and families can get involved by reaching out to the RDCRN Contact Registry or becoming involved with one of the patient organizations that partners with the Network.
For individuals and families who are undiagnosed, the ORDR offers a guide titled Tips for the Undiagnosed. This information includes a video, advice, and recommendations to other organizations that can provide assistance such as the Undiagnosed Diseases Network(undiagnosed.hms.harvard.edu/about-us/general-faqs).